Smart Supplement Reviews
Balance Of Nature Review - And Alternatives
If you’re like many Americans who find it challenging to meet the CDC’s recommendations for a healthy diet, you may have noticed the growing interest in fruit and vegetable supplements.
Sponsored Advertising Content

Is Balance of Nature Really the Best Supplement Choice?
If you’re looking to improve your health with supplements, you’ve probably come across Balance of Nature. But is it truly the best option out there?
In this detailed review, I’ll explore whether Balance of Nature lives up to the hype and share some more cost-effective and consumer-friendly alternatives that you might want to consider.
The Importance of Nutrients and the Role of Supplements
It’s a well-known fact that our bodies require a variety of nutrients to function optimally. While it’s certainly beneficial for a supplement to be doctor-formulated, I don’t believe it’s a necessity for creating a high-quality product. Doctors are trained to diagnose and treat illnesses, but they aren’t typically involved in the intricate processes of supplement manufacturing. The real challenge in modern life isn’t just knowing we need more fruits and vegetables—it’s actually consuming them in the right quantities every day. This is where supplements come into play, providing a convenient solution to help us meet our nutritional needs.
The Popularity of Balance of Nature
Balance of Nature is a household name in the supplement industry, largely due to its extensive advertising campaigns on radio and television. The brand’s visibility might suggest it’s the best option, but popularity doesn’t always equate to quality. While Balance of Nature has built a significant presence, it’s crucial to look beyond the marketing and examine the facts.
Reputation and FDA Concerns
Balance of Nature is accredited by the Better Business Bureau (BBB) and currently holds a “B” rating. This rating reflects a mixture of both positive and negative customer experiences. However, there are some red flags to consider. In 2019, the FDA issued a public warning letter to Balance of Nature, accusing the company of selling “adulterated” supplements. The FDA’s concerns were serious, citing failures in maintaining Current Good Manufacturing Practice (CGMP). Specifically, the company was criticized for not having adequate processes in place to ensure the quality and safety of their products. This raises questions about the reliability of Balance of Nature’s supplements and whether they are the best choice for your health. You can read more about this issue in the official documentation from the FDA here.
My Experience and a Better Alternative
In my personal research and testing of various top supplements, I found that Balance of Nature was not the best option for me. While it may work for some, I noticed a more significant increase in my energy levels with a different product. This alternative not only provided better results but also came at a lower cost, making it a more consumer-friendly option.
Before deciding on a supplement, I recommend doing thorough research and considering all your options. The best choice is one that meets your individual needs, is manufactured with quality standards in mind, and offers good value for your money.
Final Thoughts
Choosing the right supplement is an important decision, and while Balance of Nature has a strong presence in the market, it may not necessarily be the best choice for everyone. Be sure to explore other alternatives that might better suit your health goals and budget.
I compared some other fruit and veggie supplements designed for whole food nutrition, health, and support for overall body function (including gut health).
After comparing these products in-depth - we have listed the pros, the cons, and where to find the find the best price online.

OUR #1 CHOICE IS...
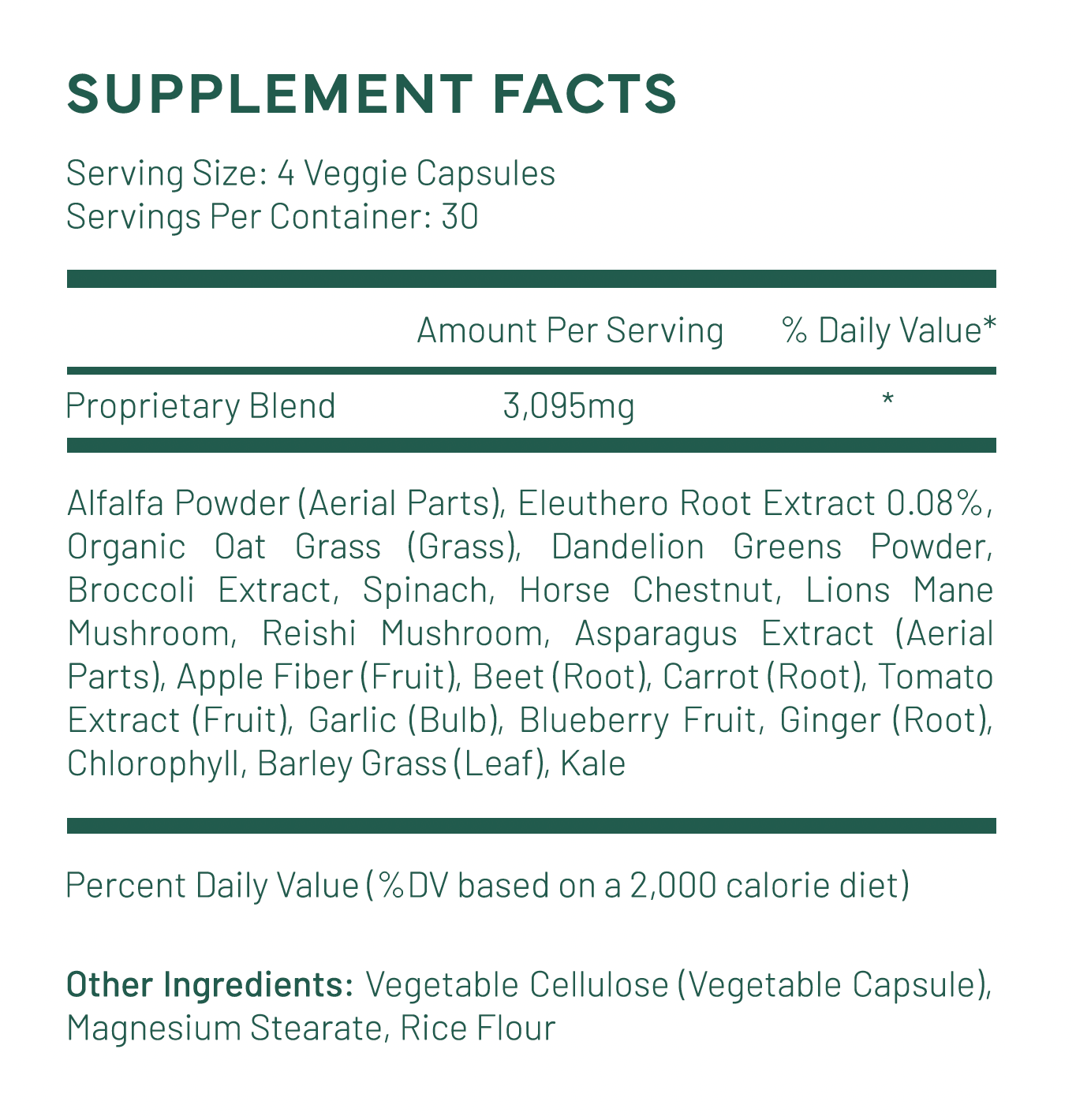
Substance - Fruits & Greens (Energy Blend)


Pros:
- Contains significant superfood blend
- Contains ingredients thought to provide energy
- Contains Chlorophyll
-
Added ginger for better absorption
-
Added mushroom blend for adaptogen value
-
**I was offered free 2 day shipping

- Formulated In The USA
- High Quality & Impactful Ingredients
- Plant-Based, Non-GMO & Vegan Friendly, Gluten Free.
- Company Has A 90 Day Money Back Guarantee

Cons:
-
Often sold out
This wonderful fruit and veggie supplement is packed full of over 20+ amazing superfoods (fruits, veggies and greens) that are sure to help your body function at it's best.
This product also contains a few notable ingredients that the others do not have:
Chlorophyll: is chemically similar to hemoglobin, a protein that is essential in red blood cells as it carries oxygen around a person's body.
Dandelion: has antioxidant properties. It may also help improve the immune system. Herbalists use dandelion root to detoxify the liver and gallbladder, and dandelion leaves to help kidney function.
Eleuthero root: is an "adaptogen" (an agent that helps the body adapt to stress). It is thought to help support adrenal gland function when the body is challenged by stress. Eleuthero has been shown to enhance mental acuity and physical endurance (give you more energy) without the letdown that comes with caffeinated products.
They also added Ginger root, which helps the body absorb all the other ingredients better!
Another huge positive is the added mushrooms, which are well known for their adaptogen properties that are thought to help the body fight off stress that causes signs of aging.
Key Points:
- 90 Day Money Back Guarantee vs. Only 30 Day From Balance Of Nature
-
Does not charge a "$24.95" member fee" like Balance of Nature does
-
Does not force you to subscribe to monthly shipments to save $$$
-
Only 4 capsules per serving vs. 6 with Balance of Nature
This product is also only half the price of Balance Of Nature


Your Privacy is Protected

Your Privacy is Protected
*Results are based on our system and do not necessarily reflect typical results from the use of these products. Please visit product websites for more information.
MY #2 CHOICE IS...
BALANCE OF NATURE


Balance of Nature Fruits and Veggies are popular whole food nutritional supplements advertised on TV and radio.
It's also very expensive. At Balance of Nature’s retail price on their website of $89.95 for Fruits and Veggies, you get 30 servings of 6 capsules. That’s $2.99 per daily dose. If you subscribe to autoshipments then you can get it for $69.95)
People with a soy allergy should avoid the Veggies capsules with added raw soybean.
Reviewers on BBB wrote that when they contacted the company they were told they were not allowed to return unopened purchases. This is poor customer service and ultimately detrimental to business reputation.
Pros:
- Vegetarian Product
- Popular Brand
- Formulated In the USA
- Has A 30 Day Money Back Guarantee
Cons:
- Charges a $24.95 one time "member fee"
-
Only offers discounts when you subscribe to monthly shipments
-
Only has a 30 day money back guarantee
-
Must take 6 capsules total daily to get a full serving
Smart Supplement Reviews does not claim to, or have any right to, any of the Trademarks relating to Balance of Nature and those which may be displayed on this page.
Results are based on our system and do not necessarily reflect typical results from the use of these products. Rankings are not intended to measure the potential efficacy of each product, and any discussion of the ingredients is for informational purposes only. Please visit product websites for more information. Advertising Disclosure
MY #3 CHOICE IS...
EARTH'S ENERGY FRUIT & VEGGIES
The ‘Fruits and Veggies’ product is 2 separate containers of 90 capsules. They are only sold together and you cannot buy them separately.
It's also MORE expensive than our $1 pick at $45 for a 30 day supply
We were a little unsure about this brand since their website leaves something to be desired. Typically legit brands build web pages that actually describe their producty vs simply trying to sell it to you in an infomercial fashion. For this reason it seems untrustworthy.
The product itself has a daily serving of 2 capsules, which is no where near enough volume to give you any level of actual nutrition benefit.
They also only have a 60 day guarantee, so they must not be too confident in their product or its quality. This brand screams red flags.
*Results are based on our system and do not necessarily reflect typical results from the use of these products. Please visit product websites for more information.
- Contains superfoods
- Cheaper than our second choice
- Ingredient panel missing from website
Smart Supplement Reviews does not claim to, or have any right to, any of the Trademarks relating to Balance of Nature and those which may be displayed on this page.
Results are based on our system and do not necessarily reflect typical results from the use of these products. Rankings are not intended to measure the potential efficacy of each product, and any discussion of the ingredients is for informational purposes only. Please visit product websites for more information. Advertising Disclosure
Disclaimer: This page may contain affiliate links to products I've researched and recommend. As an Amazon Associate I may earn from qualifying purchases at no cost to the consumer. | Information presented here is for educational and informational purposes only. The content is not intended to be a substitute for professional advice.
Copyright 2022 - SmartSupplementReviews.com
†The information on this website has not been evaluated by the Food and Drug Administration. These products are not intended to diagnose, treat, cure or prevent any disease. Your individual results may vary.

